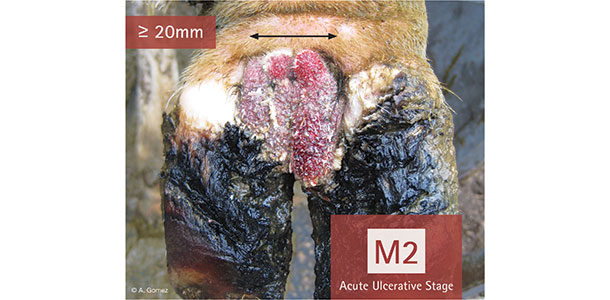
In the U.S., even on well-managed dairies, there’s a good chance that digital dermatitis (DD) is present. It is highly contagious and if left unchecked can cause painful ulcerations that often lead to lameness. Common practices for controlling DD have been limited to footbaths and topical treatment of severe lesions with no clearly established guidelines for optimal management.
Many dairy producers might be surprised to learn that DD can be effectively controlled, but it requires a slightly more sophisticated approach than the current standard of care. This article explores the tools already available that can be used on a dairy to bring DD prevalence under control.
The first step in controlling DD is to understand the epidemiology of the disease. Digital dermatitis is multifactorial with a strong bacterial component, namely Treponema spp ., which can exist in both active and cystic (dormant) forms.
Typical progression of the disease includes early, acute (ulcerative) M2 and chronic stages. In fact, DD causes changes in the shape and structure of an infected hoof before any lameness symptoms are observed.
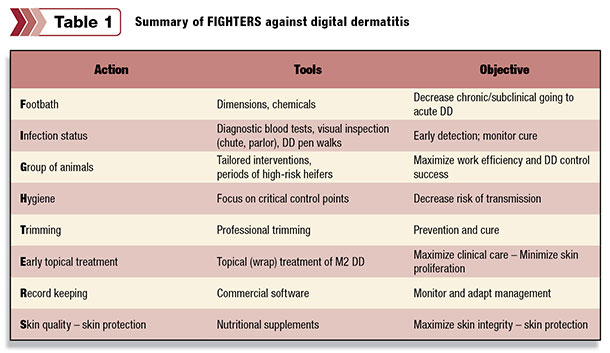
Once the disease has infected the animal, it is difficult to eliminate. It is important, therefore, to focus on minimizing new infections and decreasing the duration of acute (ulcerative) M2 cases. In order to achieve this, we rely on a set of tools available called “FIGHTERS,” which stands for:
- F ootbath
- I nfection status
- G roup of animals
- H ygiene
- T rimming
- E arly topical treatment
- R ecord-keeping
- S kin quality – skin protection
Here’s a breakdown of the FIGHTERS strategy ( Table 1 ) for controlling digital dermatitis.
Footbath
- The design of the footbath is of paramount importance to maximize the application of disinfectant solutions, decrease the amount of water used, minimize the amount of waste chemicals dumped into the environment and save money, too. Chemicals should always be used according to their labels.
An ideal footbath is 10 to 12 feet long, 1.6 to 2 feet wide, with an 11-inch step-in curb and a 4-inch minimum solution depth. Sides, sloped at 70 degrees and at a height of 3 feet, also help save solution and maintain adequate solution depth.
- The main objective of the footbath is the control of early (subclinical) and chronic lesions, avoiding the progression of these lesions into acute (ulcerative) stages. Footbaths are not a substitute for individual treatment of acute lesions.
- The appropriate frequency of footbath applications should be determined based on the needs of each herd.
Infection status
- Assessing the prevalence of the disease is the first step to quantifying the extent of the problem. The next step is evaluation of the infection status. Topical treatment applications need to be performed based on active surveillance.
Some tools such as DD pen walks, DD diagnosis in the parlor or diagnostic blood tests of active DD cases can be used to directly evaluate DD status even before lameness symptoms and chronic stages show in animals affected with the disease.
Group of animals
- The rearing period is a crucial factor for the epidemiology of the disease. Success of the DD prevention programs in the milking herd will be determined by the quality of DD prevention during the rearing period.
A recent study from our research at the University of Wisconsin has shown that approximately 67 percent of the heifers initially infected with DD during the rearing period experienced a case of DD during the first lactation.
However, animals kept free of the disease during the rearing period only experienced a case of DD during the first lactation in 13 percent of the cases.
- Precise identification of high-risk groups of animals can be achieved by evaluating the DD incidence and prevalence by days in milk or by lactation group. This is required in order to maximize the resources and the efficiency of the control programs during the rearing period and in adult cows.
Hygiene
- Digital dermatitis infection is associated with poor hygiene. The correlation between dirty environments and higher DD prevalence is widely accepted. However, even in fairly clean barns, special attention needs to be made to critical points where transmission of the disease can happen, even if the animals are exposed to problematic spots for very short periods of time.
Some examples could be when animals walk through footbaths full of manure during periods when the footbaths are not actively used, are confined to small spaces to facilitate pen cleaning activities or are exposed to manure piles dragged across alleys by scrapers.
Trimming
- Appropriate trimming can help prevent DD infections. Routine trimming of feet allows for close examination of feet for early identification and treatment of DD infections. Prevention can be achieved by removal of loose horn at the heels, wide trimming of the axial space of the lateral toe and treatment of DD lesions found during trimming (such as necrosis of the toe).
- Comprehensive trimming and foot examination programs should always take into consideration non-lactating cows, such as replacement heifers and dry cows.
Early topical treatment
- Bacterial colonization of the deeper layers of the epidermis is observed at very early stages of the disease. Over time, skin proliferation can increase as the animal reacts to the disease. Both aspects of the natural progression of the disease compromise treatment success, which is exacerbated when treatment of lesions is delayed.
Consequences of delayed treatment include increased lesion recurrence and transmission of DD to healthy animals. Only programs that include active surveillance to detect and topically treat new cases of the disease will be successful in the long run. A farm goal of zero percent presence of skin proliferation in M2 lesions at treatment can be established to recognize and monitor early treatment.
- The objective of early topical treatment is to reduce the duration of the infectious period of DD lesions and to increase cure rates. The only solution to reduce the number of active DD lesions is topical treatment.
Follow-up of the initial treatment of a DD lesion must be included in the treatment schedule. Although research efforts are being made to find non-antibiotic topical treatments, oxytetracycline (OTC) is still an effective option to treat M2 DD cases. However, working along with your veterinarian is advised when using OTC products.
Record-keeping
- The increasingly common use of on-farm management software allows for recording health events, including lameness and hoof lesions aimed at organizing future tasks (e.g., monthly number of calvings, animal movements, etc.).
These records can help determine severity and prevalence of DD infections in different groups of animals, and thus the intensity of DD control programs in specific groups of animals can be modified accordingly.
Skin quality – skin protection
- DD develops from multiple risk factors as a result of a weakening of the skin barrier, due to mechanical irritation and hyper-hydration. Improving skin integrity and enhancing immune response in the presence of bacteria (including Treponema species) that cause the disease will help provide a barrier of protection against the disease.
One way to enhance disease resistance is to provide cattle with an adequate supply of effective trace minerals, which have been shown to play a critical role in wound healing as well as maintaining the health and integrity of skin.
- Research has shown supplementing pre-calving heifers with complex trace minerals helps improve skin recovery from subclinical DD infection and maximizes the resources needed by the immune system to fight infections.
An obvious advantage is the possibility of decreasing DD prevalence even when the use of footbaths and topical treatment is limited or in cattle that are not easily handled on a regular basis, such as pastured cattle. PD
Arturo Gomez, DVM, MSc., and Dörte Döpfer, DVM, MSc., Ph.D., are both with the University of Wisconsin School of Veterinary Medicine.
PHOTO
The first step in controlling DD is to understand the epidemiology of the disease. Digital dermatitis is multifactorial with a strong bacterial component, namely Treponema spp., which can exist in both active and cystic (dormant) forms. Photo courtesy of Arturo Gomez .

Arturo Gomez
University of Wisconsin
School of Veterinary Medicine