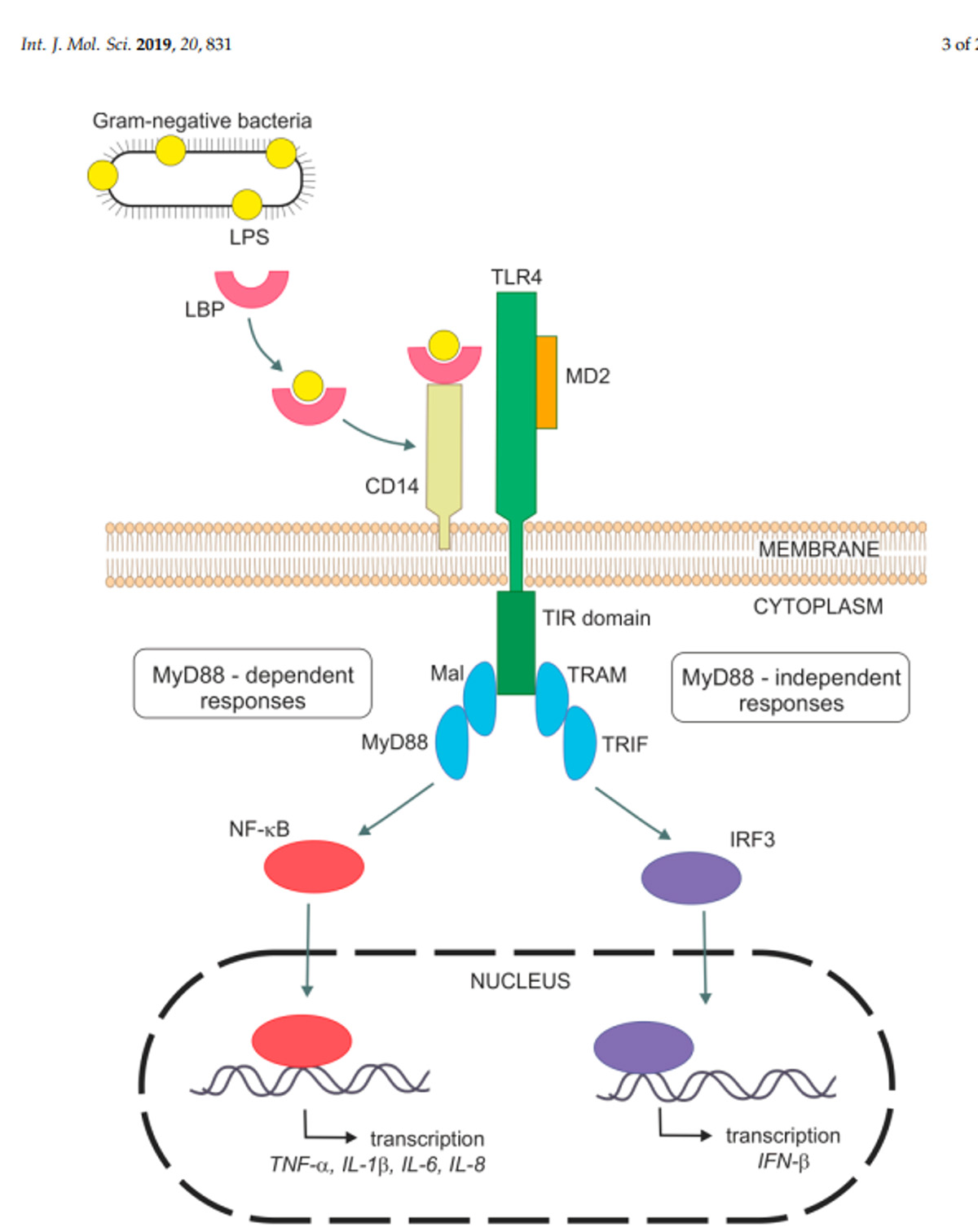
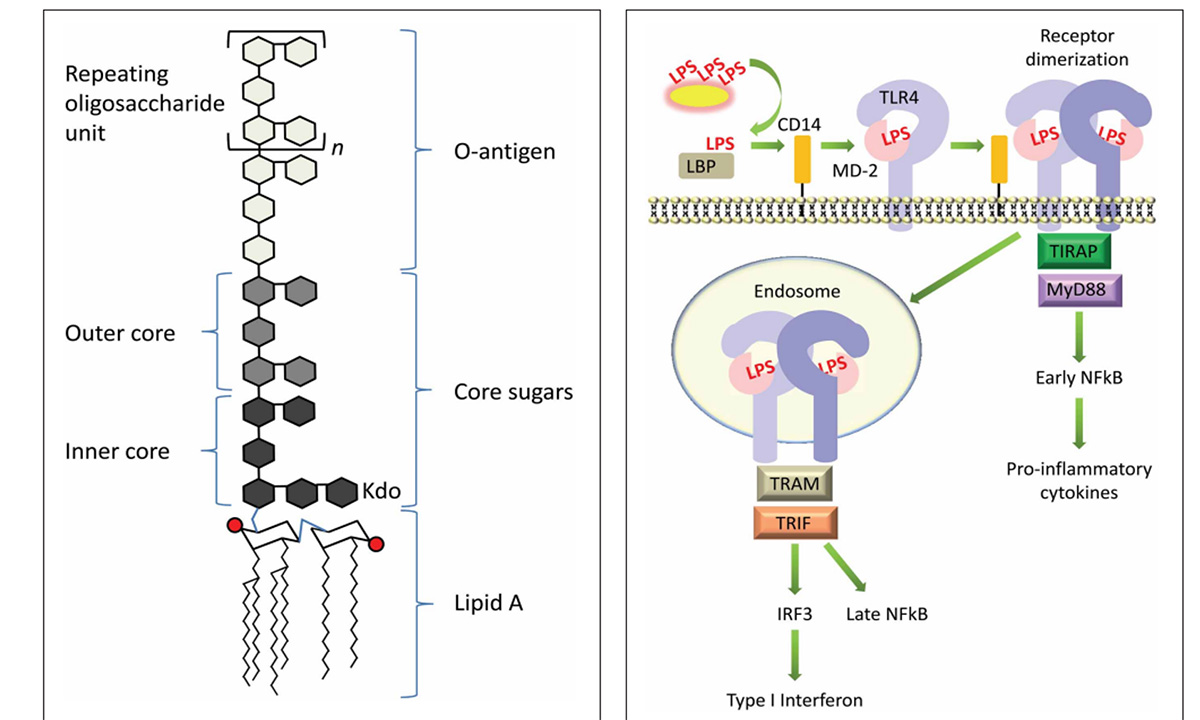
The body has several physical barriers to “outside” threats, the skin, the respiratory linings and the digestive tract. Of these three, the digestive tract faces the most potential threats, not only from what is ingested, but also from what develops in situ, through the growth of microorganisms, the toxins some produce and even the remnants of those that die within the tract. Pathogenic bacteria normally exist within the gastrointestinal (GI) tract. Common ones include species of Salmonella and E. coli. Both these bacteria are gram-negative (G-), meaning they have cell walls that will not accept that stain. These bacteria can lyse, and their cell walls are broken into segments that contain “endotoxin” made of lipopolysaccharides (LPS). Unfortunately, these LPS are quite reactive and provide strong signals to the immune system resulting in a strong inflammatory response. Lipopolysaccharides bind to TLR4 receptor sites leading to a cascade of reaction ending with the production of pro-inflammatory cytokines. Additionally, absorption of LPS have been demonstrated to reduce feed intake, which compounds the problem as animals have increased energy usage and decrease available energy from the diet, resulting in use of body lipids and potentially decreasing body proteins as well.
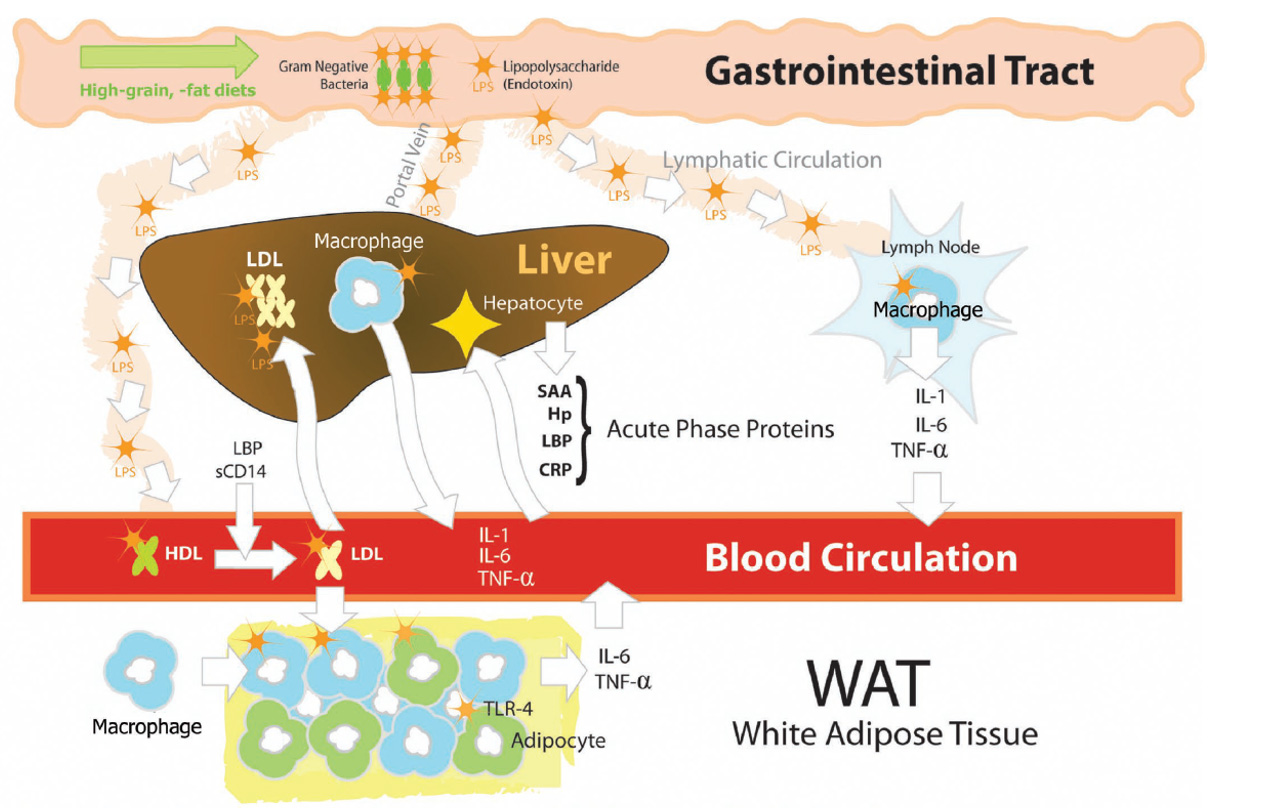 Eckel and Ametaj, JDS 2015
Eckel and Ametaj, JDS 2015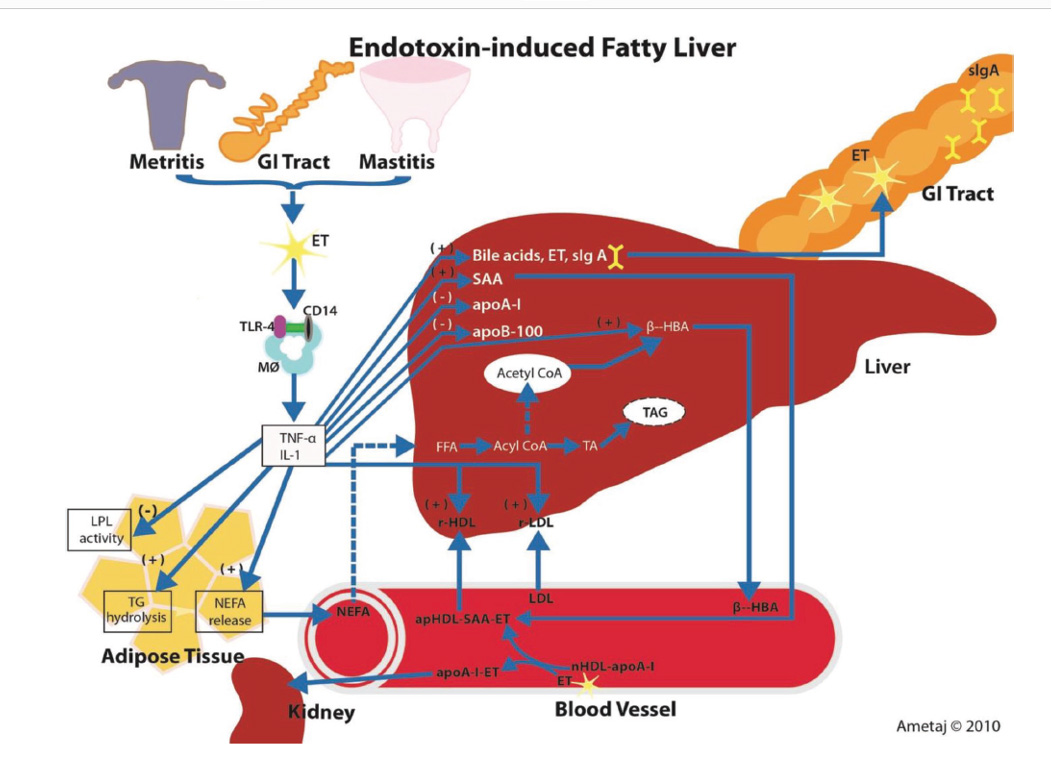
When the digestive tract is in healthy homeostasis, the number of pathogenic bacteria are held “in check.” However, disturbances in the diet or the diet itself can stimulate the growth of these bacteria; other conditions such as subclinical and clinical acidosis can result in the death and lysis of these bacteria. Diet composition can influence absorption, for example, the lipid portion of the LPS can combine with dietary fats resulting in increased absorption (Kelly et al, 2012). Additionally, conditions such as heat stress and certain mycotoxin can reduce the cell-to-cell connections and integrity of the gut, allowing increased absorption of LPS that may be present in the digestive tract.
In addition to LPS’s role in inflammation, it also has effects that can potentiate or increase the risk of other metabolic diseases. Dairy cows infused with LPS show dose-dependent reductions in serum Ca concentrations (Waldron et al., 2003). Elevated plasma LPS concentrations are associated with a decrease in blood calcium and magnesium via an increase in IL-1β (Gray et al., 2007) and may contribute to milk fever.
LPS can also affect lipid metabolism. Work by Chirivi, et al, (2022) demonstrated that LPS can reduce the antilipolytic effects of insulin, thus dysregulation of lipolysis. This may be of particular importance for transition cows that are already in need of good regulation of fat release and storage because of general negative energy balance and potential decreases in liver function.
How can the LPS threat be reduced?
Lipopolysaccharide content of the gut increases during acidosis, and managing diets to reduce clinical and subclinical acidosis is beneficial. Increasing the fiber content and reducing easily fermented carbohydrate will reduce the likelihood of excess short chain fatty acids or, in the case of clinical acidosis, lactic acid. Unfortunately, energy requirements to maximize production in either milk or muscle production may limit how much progress can be made through diet manipulation. Inclusion of buffers and some yeast products can help mitigate and increase rumen pH. Yeast can compete for soluble CHO that could be used by Strep. bovis and Lactobacillus to produce lactic acid; additionally, yeast may encourage the growth of Megasphaera eldenii, which can convert lactic acid into butyric acid. (Amin and Mao, 2021)
One can provide feed additives designed to reduce the growth of gram-negative (-) bacteria such as E. coli and Salmonella species. Yeast cell wall products have a demonstrated ability to bind the live bacteria, which reduces their ability to grow and reproduce. Feeding fructo-oligosaccharides supports the growth of probiotic or beneficial bacteria. Through their control of the microenvironment around the microvilli and through competitive inhibition, pathogenic bacterial growth can be reduced. Supplementing with certain Bacillus species can provide bactericidal compounds that directly affect gram-negative bacteria.
The growth of gram-negative bacteria and subsequent LPS production can never be eliminated. However, the effects of LPS can be mitigated through feed ingredients that help maintain a healthy homeostasis, supporting the growth of probiotic bacteria and healthy intestinal integrity along with limiting the growth and number of deleterious bacteria. Additionally, there are specific inorganic (aluminosilicates) and organic compounds (such as components of yeast cell walls) that can bind LPS and prevent both their absorption and use their ability to stimulate inflammation.
Although LPS represent a real threat to animal production, their effects can be mitigated through the use of specific feed ingredients along with practical management to reduce the risk.
Written by Volac.
Ametaj, B. N., Q. Zebeli, and S. Iqbal. 2010. Nutrition, microbiota, and endotoxin-related diseases in dairy cows. Rev. Bras. Zootec. 39:433-444.
Amin, A.A. and S. Mao. 2021. Influence of yeast on rumen fermentation, growth performance and quality of products in ruminant: A review. Animal Nutrition. 7:31-41
Eckel, E.F. and B. N. Ametaj. 2016. Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 99: 5967-5990.
Gray, C. P., T. D. St George, and N. N. Jonsson. 2007. Milk fever in dairy cattle: A novel hypothesis for immune mediated etiology. Cattle Pract. 15:277-282.
Waldron, M. R., B. J. Nonnecke, T. Nishida, R. L. Horst, and T. R. Overton. 2003. Effect of lipopolysaccharide infusion on serum micromineral and vitamin D concentrations in dairy cows. J. Dairy Sci. 86:3440-3446.
Chirivi, M., C.J. Rendon, M.N. Myers, C.M. Prom, S. Roy, A. Sen, A.L. Lock and G. A. Conteras. 2022. Lipopolysaccharide induces lipolysis and insulin resistance in adipose tissue from dairy cows. J. Dairy Sci. 105:842-855.
Maeshima, N., and R.C. Fernández. 2013. Recognition of Lipid A variants by the TLR$-MD-2 receptor complex. Cellular and Infection Microbiology. 3: Article 3.
Nova, Z. H. Skovierova and A. Calkovska. 2019. Alveolar-Capillary Membrane-Related Pulmonary Cells as a Target in Endotoxin-Induce Acute Lung Injury. Int. J. Molecular Sciences. 20: 831.
Kelly, C. J., S. P.Colgan, and D.N. Frank. 2012. Of microbes and meals: the health consequences of dietary endotoxemia. Nutr Clin Pract 27, 215-225.










